In this tutorial video we examine the intramolecular Claisen condensation known as the Dieckmann cyclization. The scheme shows a simple mechanism for the base-catalyzed aldol reaction of an aldehyde with itself.

Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
Dehydration of the Alcohol Product Effects Equilibrium.

. The mechanism of the Dieckmann condensation is the same as a. Because its a ketone to our aldehydes and youre going to get a cyclic enone. An enol Exhibit 23-3 Consider the data below to answer the following questions.
Sep 27 2020 at 1725. When a diester self-condensates the resulting product is called a cyclic β-ketoester. -CHs CH0 CH2 CH3 -CHs i What is the suitable base that should be added in the above Dieckmann condensation reaction.
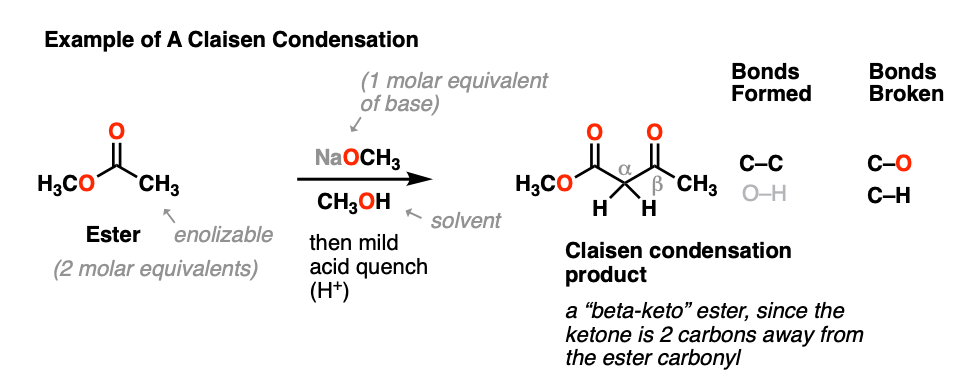
The Claisen condensation reaction is an organic coupling reaction that results in the formation of a C-C bond between either a single ester and one carbonyl compound or between two esters. The Claisen condensation is a carboncarbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base resulting in a β-keto ester or a β-diketone. In an alternative mechanism Claisen condensations in biology are often initiated by decarboxylation at the.
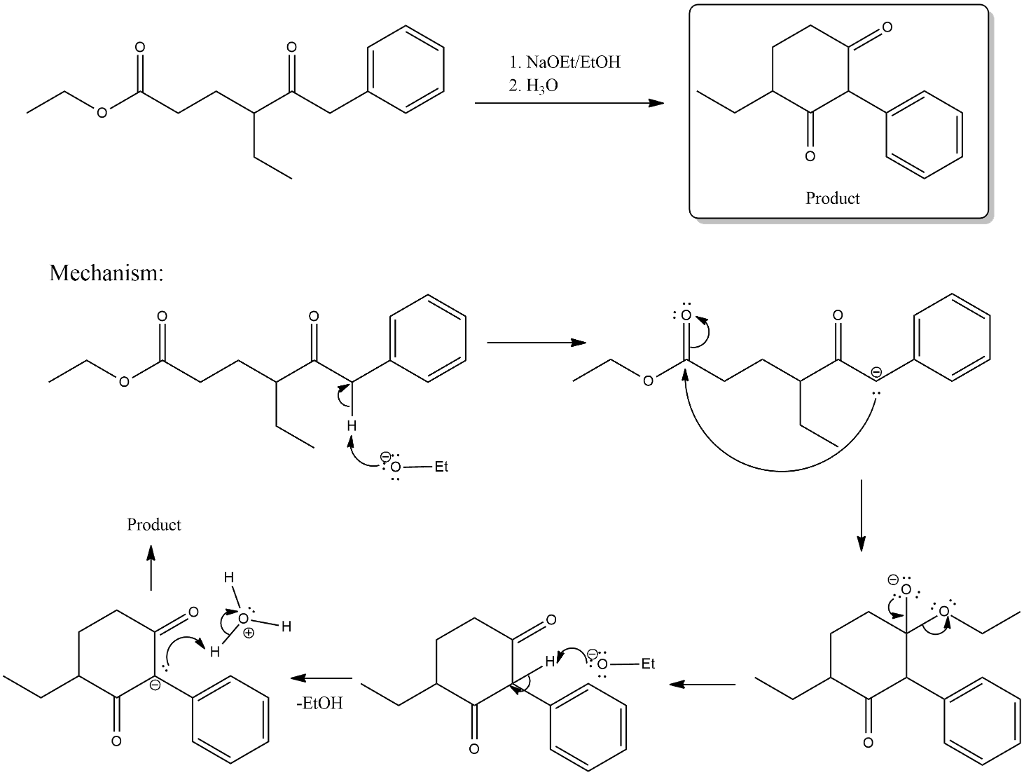
Diesters can undergo an intramolecular reaction called the Dieckmann condensation to produce cyclic beta-keto esters. It is named after Rainer Ludwig Claisen who first published his work on the reaction in 1887. Exhibit 23-6Draw the structure of the product you would expect to obtain by Claisen condensation of each of the following esters.
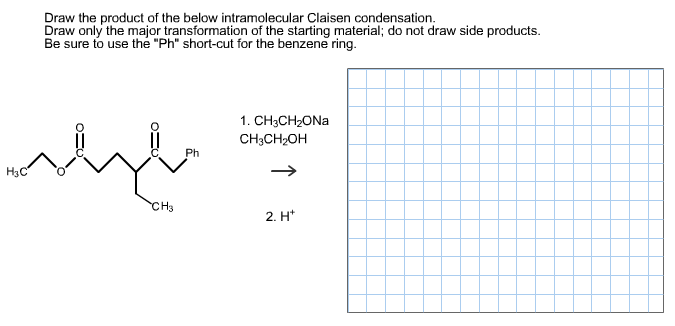
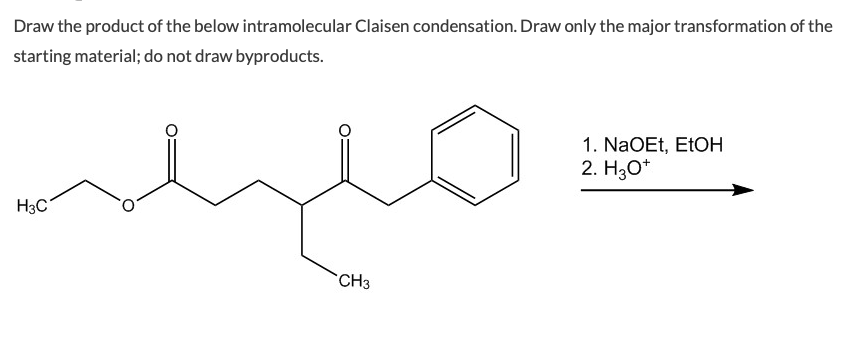
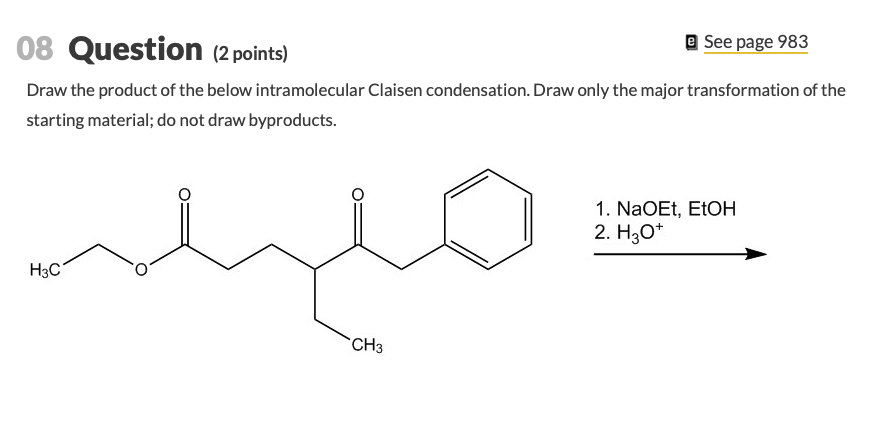
Draw only the major transformation of the starting material. Diesters can undergo an intramolecular reaction called the Dieckmann condensation to produce cyclic beta-keto esters. Do not draw side products.
Draw only the major transformation of the starting material. 2-Ethyl acetate can be prepared from ethanol as the only. Be sure to use the Ph short-cut for the benzene ring.
A Michael reaction 7. This ester does not undergo Claisen condensation because it has no a-hydrogens and cannot undergo enolization. 22-3 -- Aldol Reactions Can Also Be Acid-Catalyzed.
Cyclohexanone for example reacts with dimethylamine and acetaldehyde to yield an amino ketone. CH3CH2ONa CH3CH2OH Ph H3C H3. The reaction proceeds when a strong base is present and the product of the reaction is a beta-keto ester or a beta-diketone.
Be sure to use the Ph short-cut for the benzene ring. Show the mechanism for these reactions. R 1 and R 2 can be hydrocarbon chains of various lengths.
The reaction takes place in two steps both of which are typical carbonyl-group reactions. In the next video Im going to show you what happens for intramolecular condensations of esters. An -unsaturated aldehyde d.
A Robinson annulation d. The Claisen condensation strictly the condensation of two esters with the concomitant release of one of the constituent alcohol moieties is arguably the most important carboncarbon bond-forming reaction in biology as it is used in the biosynthesis of fatty acids polyketides and a large variety of other essential small molecule metabolites. Draw the structure of the product you would expect to obtain by Claisen condensation of each of the following esters.
On the structures provided below show electron flow with arrows in this interesting. Sep 27 2020 at 1705. User6376297 1 the beta carbon of the product is actually well sheilded by the carbonyl ester and geometry of the six membered ring so it will be very sterically costly to react at this center 2 I think amine group is a very bad leaving group so retro-michael is not much preferred.
Dehydration of the Alcohol Product Effects Equilibrium. 1-Write the complete stepwise mechanism for this reactionShow all electron flow with arrows and draw all intermediate structures. Do not draw side products.
The product of a Claisen condensation or Dieckmann cyclization is an acetoacetic ester β-keto ester EtONa EtOH EtONa EtOH Br Br C COEt O H 32 O CH3 CO 2Et O H3O. An intramolecular reaction is one in which the. A -unsaturated aldehyde b.
The product is the alkoxide salt of the aldol product. This is known as a Dieckmann Condensation or Intramolecular Claisen. What is Claisen Condensation.
The reaction proceeds when a strong base is present and the product of the reaction is a beta-keto ester or a beta-diketone. Draw the structures of the products formed when the following diesters undergo intramolecular Claisen condensation Dieckmann Condensation. Science Chemistry QA Library The following compound may undergo the Dieckmann condensation reaction an intramolecular Claisen condensation reaction when a suitable base is added to it.
The product of this reaction is. Enzymes that catalyze this reaction all. O 3H 2 O CH 3 H O Et O OEt O EtONa EtOH then H3O CO 2t O Dieckmann cyclization H 3CH 2CCOEt O 2 NaOEt O EtOH then 3H3O HCCOEt O H 3CH 2CC CH acetoi ester acetoacetic ester Claisen.
The aldol itself is then formed and it may then undergo dehydration to give the unsaturated carbonyl compound. If an ester does not undergo Claisen condensation explain why it does not. An intramolecular Claisen condensation.
22-4 -- Crossed or Mixed Aldol Condensations. A -unsaturated aldehyde b. One is the reaction between esters both having alpha hydrogens and the other is when only one of the partners has alpha hydrogens.
Et OEt 3 b. Refer to Exhibit 23-2. Draw the product of the below intramolecular Claisen condensation.
OH O Ph-CH2 CH2C-C-C-OCH3 CH2Ph 9. Reaction is Reversible. Reaction is Reversible.
If an ester does not undergo Claisen condensation explain why it does not. If both esters have ɑ hydrogens the reaction is not synthetically useful since. Draw curved arrows for the carbon-carbon bond-forming step in mechanism for this condensation reaction between two fatty acyl-thioester substrates.
A The first step is reaction between the aldehyde and the amine to yield an intermediate iminium ion R 2 C N R 2 plus water. 22-1 -- Base-Catalyzed Aldol Condensation Reactions. An intramolecular Claisen condensation b.
Draw the structure of the starting diester that forms each of the Dieckmann condensation product below. This reaction works best with 16-diesters which produce five-membered rings and 17-diesters which produce six membered rings. Draw the product of the below intramolecular Claisen condensation.
Draw the product of the below intramolecular Claisen condensation. There are two main types of crossed Claisen that we will go over in this post. Base-catalyzed aldol reaction shown using OCH 3 as base.
An -unsaturated ketone c. What B-keto ester product would be formed in the. A Claisen condensation between two different esters is called a crossed Claisen condensation.
Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. An intramolecular aldol condensation c. VIII- Consider the reaction below to answer the following questions.
Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
Claisen Condensation And Dieckmann Condensation
Oneclass Draw The Product Of The Below Intramolecular Claisen Condensation Draw Only The Major Tran

Oneclass Draw The Product Of The Below Intramolecular Claisen Condensation Draw Only The Major Tran

For The Following Reaction Draw The Major Organic Products Study Com
Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
Solved 08 Question 2 Points E See Page 983 Draw The Chegg Com
0 comments
Post a Comment